Main navigation
A specialized program of research excellence in neuroendocrine tumors
The number of individuals diagnosed with neuroendocrine tumors (NETs) has risen dramatically in the United States. To address this issue, Iowa NET SPORE researchers are exploring targeted therapies that attack tumors while leaving healthy tissue unharmed, studying the effects of common, increasingly used diabetes and weight-loss drugs on NET growth, and testing treatments in clinical trials. These approaches and therapies won't just benefit NET patients— they should also apply to other chemotherapy-resistant and/or immunologically “cold” cancers, such as prostate and breast.
The main objective of this SPORE is to advance science leading to more personalized and comprehensive care for NET patients, ultimately improving both their longevity and quality of life.
SPORE Goals
- Support innovative translational research in NETs through three new projects
- Provide support to translational investigators through interactive cores
- Enlist and encourage new translational researchers in NETs through developmental research and career enhancement programs
- Promote collaborations that yield innovative treatments and robust, evidence-based guidelines for patients with NETs and NECs

SPORE Research
Project 1: Improving immunotherapy in pancreatic NETs
Project Co-Leaders:
- Dawn Quelle, PhD (Basic Co-Leader)
- Steven K. Libutti, MD (Clinical Co-Leader; Director, Rutgers Cancer Institute)*
This multi-institutional study is tackling the critical need for better—potentially curative— treatment options for patients with metastatic pancreatic neuroendocrine tumors (pNETs). The role of B and/or plasma cells in pNET biology is unknown. Still, in other types of tumors, their presence is associated with better patient survival and improved response to immune checkpoint inhibitor (ICI) therapy.
This project will determine the importance of B/plasma cell tumor infiltrates in pNET biology and patient outcomes. Testing, for the first time, if induction of B/plasma cell tumor infiltration by cyclin-dependent kinase 4/6 (CDK4/6) blockade enhances anti-tumor immunity and efficacy of ICI therapy in pNETs.
Findings from novel mouse models and a window of opportunity trial in patients may define a new strategy to sensitize pNETs to ICI agents— promising sustained anti-tumor activity, extended patient survival, and potential cure.
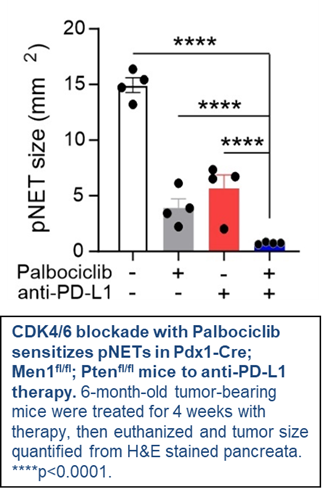
* As part of the BIG10 alliance, Holden Comprehensive Cancer Center is proud to partner with Rutgers Cancer Institute's William N. Hait Director, Dr. Steven Libutti, as a clinical co-leader on this project. Check out the Rutgers SPORE page.
Project 2: Targeting CXCR4 and Redox Metabolism for Alpha Particle Therapy of Pulmonary Neuroendocrine Tumors and Carcinomas
Project Co-Leaders:
- Douglas Spitz, PhD (Basic Co-Leader)
- Yusuf Menda, MD (Clinical Co-Leader)
Atypical carcinoids of the lung and lung neuroendocrine carcinomas (NECs) are currently incurable, with most patients succumbing to the disease within five years after diagnosis.
This project will pursue the development of an exciting new treatment paradigm for these cancers based on high-LET alpha radioligand therapy (RLT) with 212Pb Pentixather targeting the CXCR4 receptor combined with cancer cell-specific manipulations of metabolic oxidative stress.
The studies will optimize clinical imaging, therapy delivery, and dosimetry techniques and, if successful, will have a lasting impact on improving outcomes for neuroendocrine lung cancer patients.
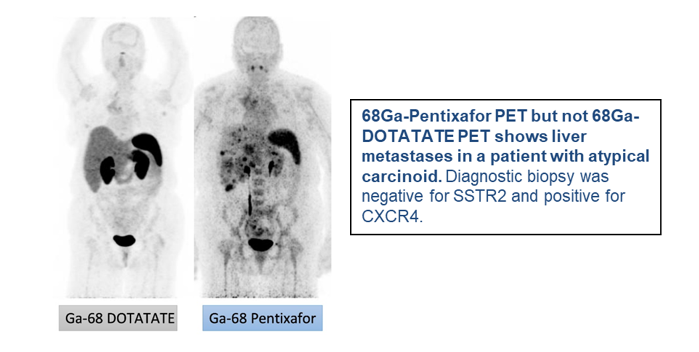
Project 3: GLP-1R/GIPR agonists in promoting gastroenteropancreatic (GEP) NET progression
Project Co-Leaders:
- Po Hien Ear, PhD (Basic Co-Leader)
- Joseph Dillon, MD (Clinical Co-Leader)
- James Howe, MD (Clinical Co-Leader)
This timely investigation will define the role and impact of increasingly used obesity and diabetes drugs, GLP-1 and GIP receptor agonists like Ozempic, on GEPNET growth. Up to 12% of Americans are already taking these drugs (known as incretin mimetics), and that number is only expected to rise.
The central hypothesis of this project is that GLP-1R and GIPR agonists selectively promote GEPNET growth depending on the expression levels of their targeted receptors. This project will be the first to 1) determine the predicted risks of incretin mimetic therapy in patients with GEPNETs that express high levels of these receptors, 2) define the biological impact, mechanisms of action, and receptor dependence of these drugs in human and mouse GEPNET models, and 3) test the possibility that current NET-approved therapies could be beneficial in blocking the tumor-promoting effects of these agents.
Results should identify which NET patients may be at risk from incretin mimetic therapy, which incretin mimetics may be taken safely, and how unwanted incretin mimetic effects might be ameliorated.
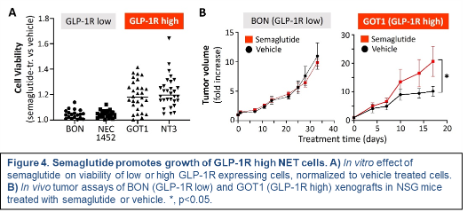
Additional research support
Administrative Core
Core Directors:
The Administrative Core provides the main organizational structure and oversight of all SPORE activities. The overall goal is to support innovative, collaborative, and rigorous research in NETs and oversee the translation of our team’s findings into improved diagnostic and therapeutic options for NET patients.
Biospecimen Core
Core Directors:
The Biospecimen Core provides a coordinated, centralized, and dedicated service for procuring, processing, and annotating high-quality biospecimens from patients in our Iowa NET Registry and SEER Residual and Iowa Virtual Tissue Repositories. The specimens collected and maintained herein constitute the largest and most well-characterized resource of NET and NEC tumor tissue in the United States. This work is fundamental for our investigators and programs to adequately tackle clinically relevant scientific questions relating to these perplexing tumors.
Clinical Core
Core Directors:
- Joseph Dillon, MD
- Muhammad Furqan, MD
The overarching goal of the Clinical Core is to serve as a bidirectional link between patients with neuroendocrine tumor (NET) or neuroendocrine carcinoma (NEC) and advances in research, diagnosis, treatment, long-term care, and, ultimately, disease prevention. Toward this end, 98% of patients attending the Iowa NET Clinic participate in the IRB-approved NET Registry, which currently includes over 2,400 subjects who can be directly contacted if a clinical trial is being developed for which they may qualify. Additionally, we are leading a multi-institutional collaboration to create a de-identified database that will include over 7,000 subjects, facilitating population science and patient-reported outcomes research.
Career Enhancement Program (CEP)
Program Directors:
The goal of the CEP is to guide the development of creative translational scientists experienced in multidisciplinary research for future leadership in NET research. Providing ample opportunities for training and career enhancement is a top priority of the University of Iowa NET SPORE and our scientific community. The CEP is critical to our long-term commitment to recruiting bright, energetic new investigators into NET translational cancer research and infusing the field with innovative talent and ideas.
Developmental Research Program (DRP)
Program Directors:
Our DRP seeks to bring new ideas and innovative techniques to bear on the diagnosis, curative therapy, and the ultimate prevention of NETs. We anticipate that support of developmental research projects will continue to generate new hypotheses to test in SPORE-sponsored projects or through peer-reviewed external grant support. The long-term goal of the Iowa NET SPORE program is to translate the findings of developmental projects into an improved length and quality of life for patients with neuroendocrine tumors.

Multi-PI Team
Jim Howe, MD; Dawn Quelle, PhD; Yusuf Menda, MD.
NET SPORE News
- Po Hien Ear and colleagues published in Endocrine Oncology (https://pubmed.ncbi.nlm.nih.gov/41312422/): Examining glucagon-like peptide-1 receptor (GLP-1R) expression across neuroendocrine neoplasms (NENs), the research identified unexpected patterns of GLP-1R expression in specific NEN subtypes. These findings are clinically significant as use of incretin mimetic medications that target GLP-1R, such as Ozempic, continues to rise among U.S. adults. Given the potential oncogenic effects of these agents in tumors with high receptor expression, this work has important implications for patient care and risk assessment.
- North American Neuroendocrine Tumor Society (NANETS) Symposium 2025, Austin, TX.
Iowa NET SPORE speakers:
Dawn Quelle, From Setbacks to SPORE: Advancing NET Research Through Teamwork and Tenacity (keynote address)
Andrew Bellizzi, Overview of IHC NEN Evaluation
Joseph Dillon, Clinical integration of GLP-1 into patient care - risks and clinical integration
Po Hien Ear, GLP-1R expression levels in NET TMAs and NET models for testing GLP-1
Michael O’Rorke, Quality of Life and Care Experiences in a U.S. Multi-Institutional Neuroendocrine Tumor Cohort
Erik Mittra (SPORE External Advisor), Moderator of Featured Abstracts: Molecular Characterization and Interventional Strategies in NETS
Awards to former Iowa NET SPORE trainees:
Rising Star Award- Jess E. Maxwell, Assistant Professor, Department of Surgical Oncology, University of Texas MD Anderson Cancer Center (prior mentee of James Howe)
Diversity, Equity & Inclusion Award- Udhayvir Singh Grewal, Clinical Assistant Professor, Department of Hematology and Oncology, Winship Cancer Institute of Emory University (prior mentee of Chandrikha Chandrasekharan)
- Neuroendocrine Tumor Research Foundation (NETRF) Symposium 2025, Boston, MA.
- Poster presentation – Tao Xu (mentor Michael O’Rorke), Quality of Life and Care Experiences in a U.S. Multi-Institutional Neuroendocrine Tumor Cohort
- Speaker – Andrew Bellizzi, Variable GLP-1 Receptor Expression Across Diverse Neuroendocrine Neoplasms: Implications for Incretin Therapies
- Moderator – Dawn Quelle, Session 3: Tumor Microenvironment
- 2025 Developmental Research Project (DRP) and Career Enhancement Project (CEP) Awardees
DRP
Samuel G. Katz, MD, PhD (Associate Professor, Department of Pathology, Yale School of Medicine) – “Development of chimeric antigen receptor (CAR) T cells targeting MET for neuroendocrine tumors (NET).” Co-PI: Pamela L. Kunz, MD (Professor, Department of Medicine / Medical Oncology, Yale School of Medicine)
Dustin Bosch, MD, PhD (Assistant Professor, Department of Pathology, University of Iowa Health Care) – “Microbiome contributions to the development and progression of ileal well-differentiated neuroendocrine tumors.” Mentor: Andrew Bellizzi, MD (Professor of Pathology, University of Iowa Health Care)
CEP
Udhayvir Grewal, MD (Assistant Professor, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University) – “Plasma epigenome profiling to inform clinical response to radioligand therapy in gastroenteropancreatic neuroendocrine tumors.” Mentors: Daniel M. Halperin, MD (Associate Professor, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University) and Jacob C. Berchuck, MD (Assistant Professor, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University)
Ahmad Shariftabrizi, MD (Clinical Assistant Professor, Interim Division Chief of Nuclear Medicine - VAMC, Division of Nuclear Medicine, University of Iowa Carver College of Medicine) – “Covalent-binding radioligands for enhanced tumor retention in neuroendocrine tumors.” Mentors: Yusuf Menda, MD (Professor of Radiology/Nuclear Medicine, Division Chief of Nuclear Medicine, Director of Theranostics Program, University of Iowa) and Christopher Pigge, PhD (Associate Professor of Chemistry, University of Iowa)